Therapeutic Guidelines are written principally for prescribers to provide clear, practical, succinct and up-to-date therapeutic information, for the management of patients with specific conditions.
Therapeutic Guidelines cover common disorders in clinical practice. Topics and sections are arranged according to diagnostic entities. Each section gives sufficient surrounding information to orient the reader, followed by succinct and explicit recommendations for therapy.
Therapeutic Guidelines are based on the latest international literature, interpreted by some of Australia’s most eminent and respected experts, with input from an extensive network of general practitioners and other users. The information is independent and unbiased and is a distillation of current evidence and opinion.
- Independence
- Expert groups
- Frequency of review
- Review process
- Basis for recommendations
- Drug use in pregnancy and breastfeeding
- Referencing
- Evaluation and feedback
Independence
Therapeutic Guidelines is an independent not-for-profit organisation. Its aim is to promote the quality use of medicines, and it does this through the writing, publication and sale of Therapeutic Guidelines.
Independence is a critical issue for Therapeutic Guidelines because its reputation depends on the integrity of its content. Therapeutic Guidelines is financially independent, with funding generated solely from sales and subscriptions to the Guidelines.
There are two key aspects of Therapeutic Guidelines’ independence. Firstly, Therapeutic Guidelines is independent of any form of government or commercial sponsorship including the pharmaceutical industry. Secondly, Therapeutic Guidelines has a strict policy on conflict of interest for staff, Directors and expert group members.
Conflicts of interest are minimised by careful selection of expert group members. Any remaining conflicts are declared and managed during the guideline development process in accord with Therapeutic Guideline’s conflict of interest policy. A register of declared interests for expert group members can be found on the relevant guideline page.
Expert groups
The content of Therapeutic Guidelines is developed by expert groups in collaboration with an in-house editorial team, including an Editor, Senior Editor and Chair.
Therapeutic Guidelines convenes expert groups that include local clinicians, who are leaders in their therapeutic area, and represent diversity and experience across various healthcare settings. Each expert group comprises approximately 15 people, including a chair, one or two editors, experts in relevant medical specialties, a general practitioner, a junior hospital doctor, and a pharmacist. For each guideline revision, there is a reasonable balance of new members, and members who have been on the expert group before to bring new perspective and breadth to the network. Depending on the subject matter, the expert group may also include experts from other areas, such as nursing, physiotherapy and nutrition.
See a list of expert groups by guideline.
Frequency of review
Therapeutic Guidelines prides itself on being one of the few guideline development organisations that commits to revising topics on a regular basis. Each Guideline undergoes a full review approximately every four years, which is the time frame in which evidence and practice significantly shifts for most clinical areas.
To address seminal changes in the evidence or in clinical practice between full guideline reviews, targeted updates are made with an expert group convened to consider a specific issue, a rolling expert group, or by liaising with individual experts as needed.
The editorial team collates feedback on the current content and engages with the expert group and an evaluation network to identify significant changes in the evidence base or in clinical practice in relation to the scope of the guidelines – this guides the schedule of review and can prompt targeted updates.
The Updates page includes detail on guidelines currently under revision or planned for publication soon, as well as a summary of major changes made in each release.
Review process
The process for revising Therapeutic Guidelines is illustrated in the diagram below. Full guideline revision usually requires an initial planning meeting plus six working meetings, held over approximately 9 to 12 months, while targeted updates can be made much more swiftly.
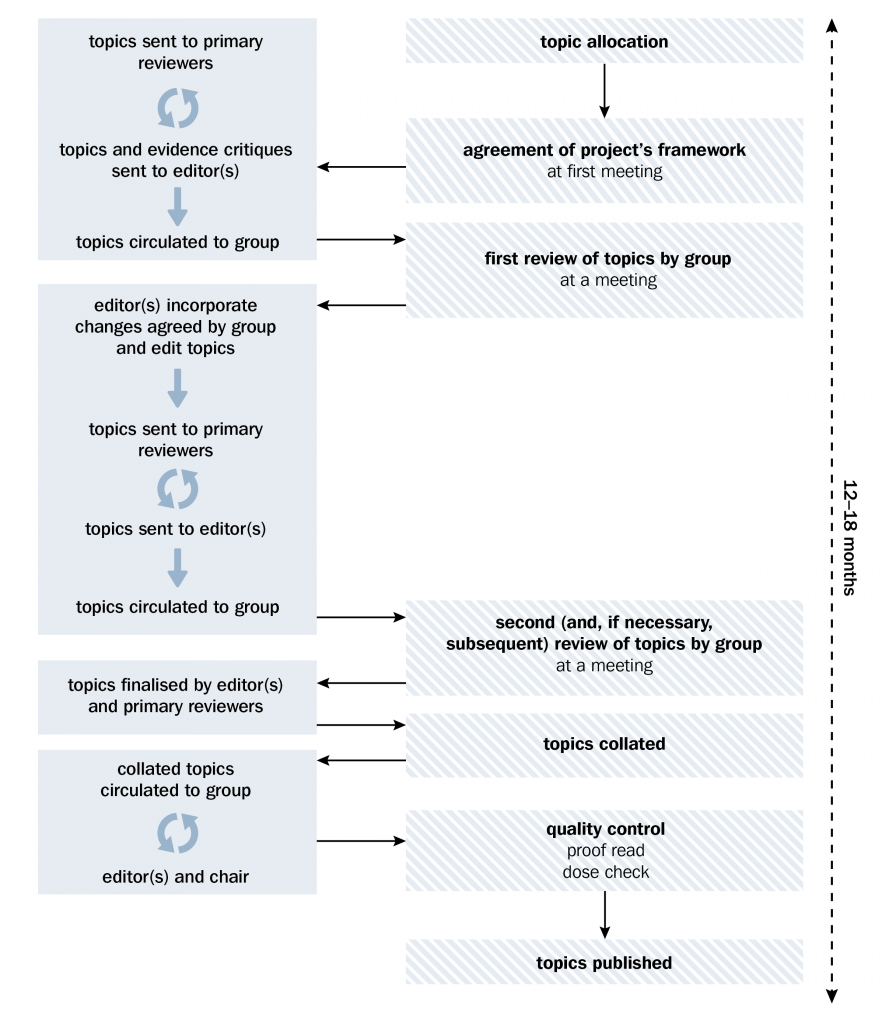
The chair, senior editor and editor(s) are employees of Therapeutic Guidelines and play pivotal roles in the revision and development of content for publishing. The chair ensures the project proceeds harmoniously and that consensus is achieved for all recommendations. The editor manages the project, and shapes the tone and brevity, to provide clear and concise advice that is evidenced based, practical and integrates with other content in Therapeutic Guidelines.
The expert group discusses the scope of the Guideline and feedback on current content, taking into account the prevalence of conditions. Sometimes advice on uncommon but serious diseases will be included. Decisions on scope are often influenced by feedback from users. Usually, guideline review involves updating existing guidelines, but it is not uncommon for expert groups to create new topics or to expand the scope of the current topics.
The expert group considers the evidence since the previous revision, and decides what changes should be made, considering the practical application of recommendations in practice. The process occurs over a series of meetings, considering issues that require debate by the expert group (eg when evidence is poor or conflicting or not practical in all settings) until consensus is reached for each significant change.
The finished manuscript is the result of detailed scrutiny, collaboration and revision by the entire expert group, so topics cannot be attributed to one author. The expert group is therefore the author of each topic and the Guideline overall.
Basis for recommendations
The relevance and strength of the scientific evidence for the effectiveness of any given treatment are fundamental to the development of the content in Therapeutic Guidelines. In clinical areas where there is strong evidence, there can be a reasonably high level of certainty about which treatment(s) to recommend. However, to make sure that the advice is clinically relevant and applicable, the information must be contextualised to reflect the reality of everyday clinical situations—co-morbidities, risk factors, patient characteristics and affordability of treatment options are examples of important factors that must also be taken into consideration.
‘Evidence-rich’ areas are in the minority in clinical practice, so a large proportion of the material developed for Therapeutic Guidelines covers areas for which there is little published evidence. In these ‘evidence-poor’ areas, the pathophysiology of the disease, the clinical experience of expert group members, and the adverse effect profiles, long-term safety data, and cost, of the therapeutic options become more significant in making recommendations. When drug recommendations are made, the order of preference is indicated by numbering them.
Rather than assigning levels of evidence to statements or grading recommendations, the Guidelines use the surrounding text to indicate the relevant evidence. This approach gives more opportunity to communicate the reason for making recommendations, particularly in ‘evidence-poor’ areas.
Drug use in pregnancy and breastfeeding
The categories for drug use in pregnancy included in Therapeutic Guidelines are from the Prescribing medicines in pregnancy database on the TGA website www.tga.gov.au/hp/medicines-pregnancy.htm.
Advice on drug compatibility with breastfeeding is updated by two expert reviewers, who apply a standard terminology to describe compatibility for individual drugs, considering current literature and a range of evidence-based resources. The reviewers also consider a drug’s therapeutic and toxicity profile and its physicochemical properties, the route of maternal administration, and the likely duration of maternal therapy.
Referencing
Key references are published in Therapeutic Guidelines in support of:
- major changes from previous text
- drug recommendations not within the product information
- controversial statements
- numbers (eg in tables and text where the source of the information is not readily apparent)
- any statement prefaced with ‘evidence shows’ (or similar) or citation of a specific trial.
Due to limited space, print versions of the Guidelines only include references for further reading or key references that might be helpful to the reader (ie major new and pivotal studies).
Evaluation and feedback
TGL liaises with an Evaluation Network of approximately 200 users (including general practitioners, junior hospital doctors, health academics, pharmacists and students) to actively solicit feedback on the Guidelines. Participants in the Evaluation Network are provided with eTG complete, free of charge. TGL also contacts numerous stakeholders, such as the Australian Commission on Quality and Safety in Health Care and NPS MedicineWise, at the beginning of each project to ask for feedback and facilitate collaboration.
We regularly receive feedback from users that helps to shape and prioritise content development. Users are encouraged to submit feedback using the ‘envelope’ button in each topic or by emailing comments directly to feedback@tg.org.au.
Accrued feedback on the previous version is collated and passed on to the expert group for consideration when revising the text. Feedback is an important signal for targeted updates to the content in between scheduled revisions. The decision to update a topic in between editions is made by the Editorial team in collaboration with the previous expert group.
Therapeutic Guidelines also liaises with an Evaluation Network of approximately 200 users (including general practitioners, junior hospital doctors, health academics, pharmacists and students) to actively solicit feedback on the Guidelines. Participants in the Evaluation Network are provided with a complimentary subscription to Therapeutic Guidelines. Therapeutic Guidelines also contacts numerous stakeholders, such as the Australian Commission on Quality and Safety in Health Care and NPS MedicineWise, at the beginning of each project to ask for feedback and facilitate collaboration.
Occasionally, new guidelines are developed to address an unmet need (eg size of health burden, cost, lack of evidence) or in response to an expressed need by users, medical organisations or government.
Updated: September 2021

